FREE ACCESS: Internal Document Shows 19 Post-Vaccination Deaths in Jamaica, Despite 11 Publicly Reported A Day Earlier
Nine Already Ruled Not From Vaccine. Two "Indeterminate".
Jamaica’s health ministry made a commitment to the press on April 14 that “we will be transparent in our reporting” of adverse events.
But I’m hard-pressed to find one occasion since that day that it has volunteered any post-vaccination surveillance data without being prompted.
Even when journalists ask questions on the topic, the health ministry isn’t always completely forthcoming, as you’ll see in this 18º North exclusive report.
Sometimes they’ll share information on an individual basis as one representative recently did with me. That information has formed the basis of this report.
Because of the public-health implications, 18º North is making this article free today, having first made it available to paid subscribers last week. It’s been modified to provide new information and greater clarity for readers based on feedback.
Help me to continue this kind of reporting that pushes the ministry for more routine and widely-dispersed information on vaccine safety surveillance by becoming a paid subscriber of 18º North. It’s US$11.50 a month or US$115 a year, including sales tax.
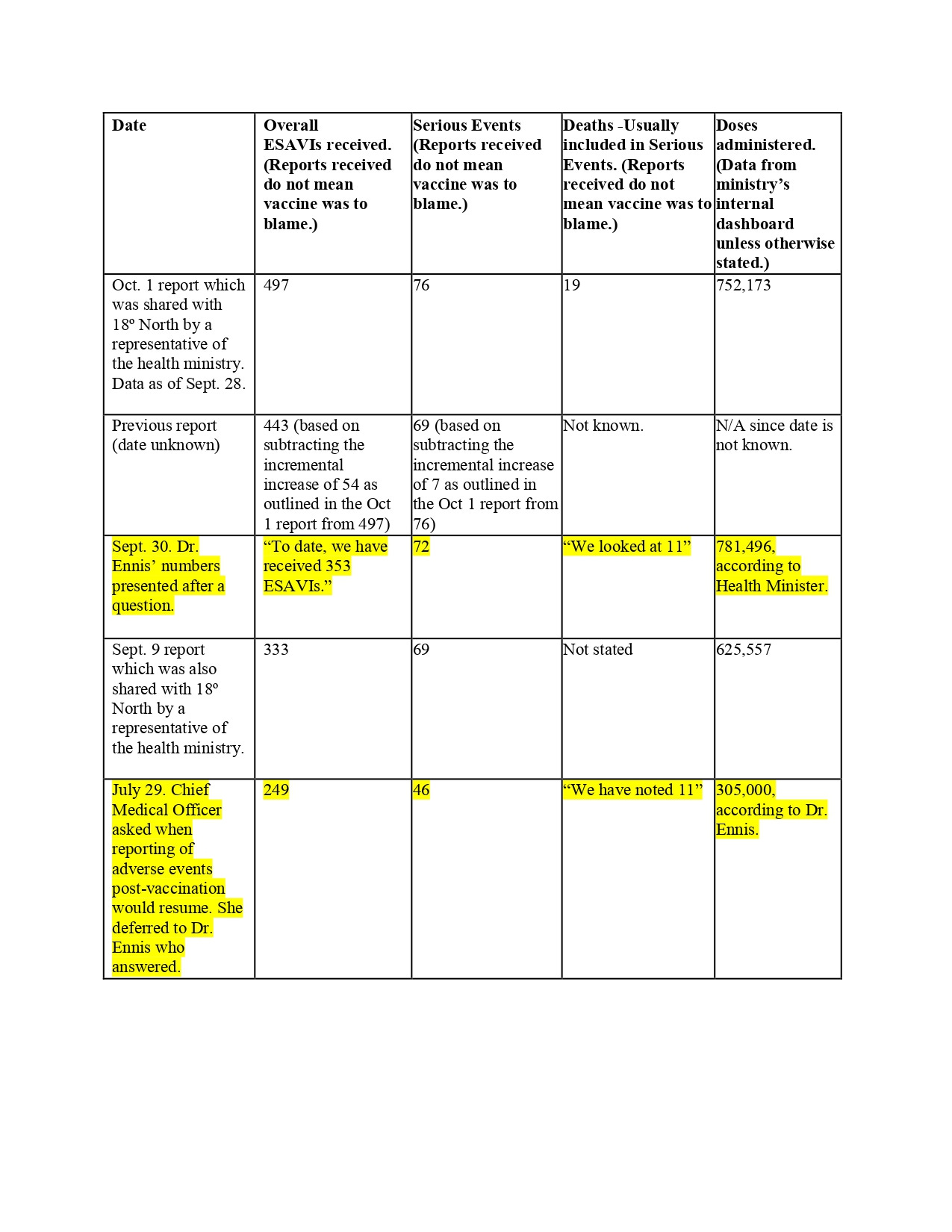
A day after the head of Jamaica’s vaccination program Dr. Melody Ennis told journalists on Sept. 30 that “we looked at” eleven deaths post-vaccination, an Oct. 1 document shared with 18º North by another member of the health ministry shows that there were 19 such deaths.
The 19 deaths pale in comparison to the country’s almost 2,300 Covid fatalities, and don’t necessarily mean that the vaccine was to blame. (Already nine were determined to not have been caused by the vaccine.)
But the fact that there is a significant difference between the number of deaths that have been publicly-stated and the figure that’s internally known calls into question whether the most up-to-date and complete information on post-vaccination safety surveillance is being shared with the public by the Ministry of Health & Wellness, as is recommended by the World Health Organization (WHO).
What The Internal Report Shows vs. What The Public Was Told A Day Earlier
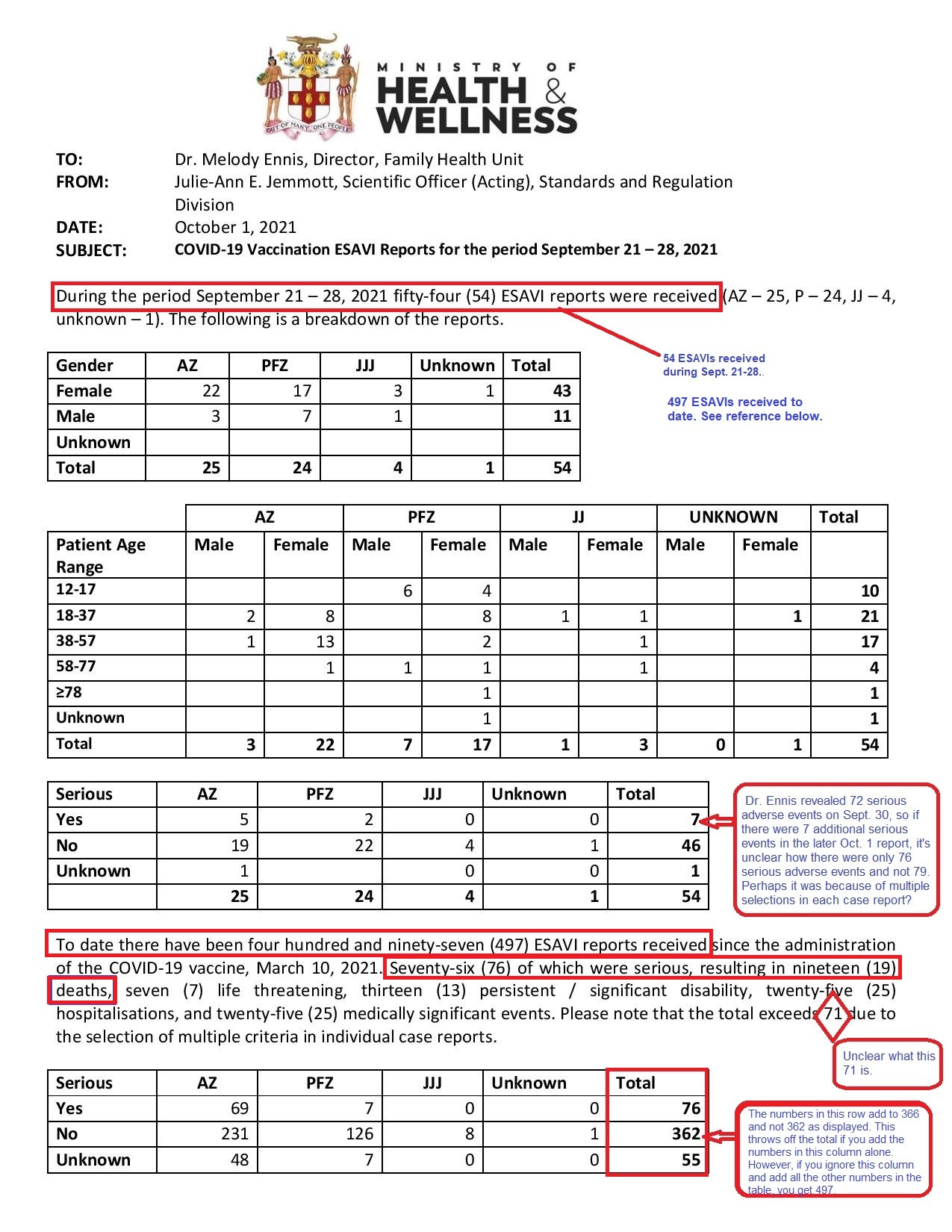
The Oct. 1 report, which covered Sept. 21-28, came from the Standards and Regulation Division within the health ministry and was addressed to Dr. Ennis.
The report contains several mathematical discrepancies and some fuzziness in the numbers owing to the selection of multiple criteria in the individual case reports, but the overall findings are still telling.
For example, the text outlines that since the Covid-19 vaccine rollout on March 10, the ministry had received 497 reports of events supposedly attributable to vaccination or immunization (ESAVI).
While the numbers in the column add to a different total, 493, either figure would mean that the ministry was close to recording almost 500 reports of adverse events, and yet Dr. Ennis said at a virtual press conference a day earlier on Sept. 30 that “To date we have received 353 ESAVIs.”
Dr. Ennis was responding to a question from journalist Roxroy McLean of The STAR who asked for “the number of serious adverse reactions to the vaccines as well as the number of persons who have died after getting the jab.”
Dr. Ennis answered that of 353 adverse events, “we have classified 72 as being serious” and “we looked at eleven persons who would have died after receiving the vaccine.”
Then in a follow-up conversation about vaccination data, she referred 18º North to another member of the health ministry, who shared the Oct. 1 ESAVI report that outlined higher figures.
That report stated there had been 76 serious adverse events, “resulting in nineteen (19) deaths.”
Video: Dr. Melody Ennis addresses journalists at a press conference Sept. 30 on adverse events post-vaccination.
When 18º North asked Dr. Melody Ennis how come she disclosed only 353 ESAVIs and eleven deaths on Sept. 30, she said, “I reported what I had…It’s a different report.”
“I would have reported on a previous report the week before or the week before that, and now we get an updated report, so what is the challenge? The report you have is at the 1st of October, is that not so!” she retorted.
Based on the Oct. 1 internal document, 54 incremental ESAVIs were received between Sept. 21 - 28, which would mean that the previous report would have contained 443 adverse events and not the 353 that Dr. Ennis reported on Sept. 30. The possibility exists, then, that she was quoting from a report the week before, but then that would raise a different question as to whether there was an acceleration of these kinds of reports.
18º North asked Dr. Ennis by email if it was a matter that within the last few weeks of September the country had an additional 144 ESAVIs in order to move from 353 to 497, and an additional eight deaths reported in order to move from eleven to 19, but she didn’t respond.
When pressed as to the date of the report from which she was pulling information, Dr. Ennis said she didn’t have it to hand, and she didn’t respond to an email sent to her on Oct. 5 asking the same question.
Dr. Ennis first revealed the eleven deaths on July 29 and said that this was out of 305,000 doses of vaccines administered. However, even after vaccine doses had more than doubled to 1781,496 by Sept. 30, Dr. Ennis still maintained that the ministry had “looked at” eleven deaths. (Because Dr. Ennis has not given the exact date of the adverse-event report from which she was pulling information, the corresponding number of doses administered for that specific date could not be ascertained.)
Four Vaccine-Product Related Reactions
The only figure that was consistent in both Dr. Ennis’ Sept. 30 disclosure and the Oct. 1 report were 17 closed cases that were “fully reviewed and classified.”
Dr. Ennis had said these 17 were from the 72 serious cases, but she didn’t explicitly explain that four of them were deemed to have been a “vaccine-product related reaction” as outlined in the report.
Instead, at the press conference, she referred to dizziness and “a few” hospitalizations due to allergic reactions or palpitations.
She also disclosed that nine of the eleven deaths were found to be coincidental, which, she had explained previously, means “they were not caused by the vaccine.” Two were 2indeterminate temporal, she said, which the ministry had stated, means there is no established link to their vaccination.

Dr. Ennis and Julie-Ann Jemmott of the Standards and Regulation Division were later asked about the nature of these four vaccine-product related reactions detailed in the Oct. 1 report, and about some of the mathematical discrepancies, but neither of the two responded to the email.
18º North also asked Dr. Ennis if, by disclosing only eleven post-vaccination deaths, the ministry was sticking to a policy it had previously announced of disclosing information only after the cases had been fully investigated. However, she didn’t answer that question either.
She also didn’t respond when asked whether, outside of the 11 deaths already investigated, causality had yet been established in the eight additional deaths. Dr. Ennis had said in the past that causality is usually determined by a committee that includes “clinicians with expert training in the particular field that we are considering at the time.”
The issue of side effects has been one reason members of the public have cited as to why they don’t want to take the vaccine. In Jamaica, vaccine hesitancy is already one of the highest in the world, with less than a third of persons saying they would take a Covid-19 vaccine if it was offered to them for free, according to a Gallup poll conducted last year. About 280,000 expired doses of AstraZeneca vaccines have also been discarded due to a lack of uptake.
When told about the two sets of numbers from the ministry a day apart, Dr. Kevin Harvey, Head of School of Public Health & Health Technology at the University of Technology (UTech) in Jamaica maintained what he told 18º North earlier this year that the ministry should be more forthcoming about post-vaccine deaths and report them in the same way that Covid-19 deaths are reported, which often include deaths under investigation.
“Giving as much information as you can is really the best way to go,” he said. “If the public perceives that the government or the ministry is hiding something, then it could also lead to further mistrust.”
How The Ministry Has Communicated Adverse Events Post-Vaccination
3The WHO’s Global Manual on Surveillance of Adverse Events Following Immunization, advises countries that when communicating with the media, “waiting for the conclusion of an investigation is rarely possible.”
“Information may need to be disseminated early and often, and it is vital to be honest about what is known and what is not known, and to avoid being evasive and unresponsive.”
The WHO’s Safety Surveillance Manual on Covid-19 Vaccines also states, “Communication that is transparent, timely, empathic and acknowledges uncertainty can help boost people’s trust in health authorities, which in turn can positively influence people’s willingness to be vaccinated.”
However, though the health ministry has repeatedly said it takes its guidance from the WHO, and though the Chief Medical Officer Dr. Jacquiline Bisasor-McKenzie committed to the Jamaica public on April 14 that “we will be transparent in our reporting” on adverse events, it has put forward a presentation only once to the press on the topic, and that was on the same day back in April. Every other time the topic has come up, it’s been prompted by a question from a journalist. Additionally, even as the ministry has held two press conferences since Sept. 30, it has said nothing publicly about the updated number of 19 post-vaccination deaths. (The U.S., U.K. and Canada report their summaries of adverse events weekly and have much higher rates of vaccination than Jamaica. Data from the Vaccine Adverse Event Reporting System in the U.S. are also available on demand.)
Key Numbers From The Oct. 1 Report
Of the 54 ESAVIs received between Sept. 21 - 28, there were 25 associated with the AstraZeneca vaccine, 24 with the Pfizer-BioNTech, four with the Johnson & Johnson (J&J) and one unknown.
Females reported more side effects than males, regardless of brand. For every report from a male, there were four from a female. However, in children aged 12 to 17, boys were more affected. Of the ten reports for the week, six were boys and four were girls.
Almost 90% of the complaints came from those aged 12 to 57, but based on the ministry’s internal dashboard, those in this age range also made up the majority of persons vaccinated during this period and in the immediate weeks before.
Counting all ESAVIs as of Sept. 28, the most common side effects were dizziness, headache and injection site pain. There were also complaints about pyrexia (fever), nausea and blood pressure being increased.
Proportionate to the doses administered, there were more adverse events reported after the Pfizer than the other brands with one report for every 1,448 doses compared to one for every 1,504 doses of AstraZeneca, and one for every 3,260 doses of J&J.
Dr. Ennis explained on Sept. 30 that because children and adolescents received the Pfizer they were more likely to complain about the common side effects.
For the serious adverse events, however, there were proportionately more reported after the AstraZeneca. There was one report received for about 7,600 doses of that brand compared to one in roughly 29,000 doses of the Pfizer. All 19 deaths also occurred after the AstraZeneca. There were no serious events recorded after the J&J, although there had only been about 26,000 doses administered of that brand at that time, while there were almost 203,000 doses of the Pfizer and more than 523,000 of the AstraZeneca.
Despite these findings, several experts consulted by 18º North say these data points shouldn’t be relied upon to derive side effect rates or to compare the safety profile of the vaccines because many factors can influence the number of reports received. The Pfizer and J&J were also only rolled out this summer compared to the administration of AstraZeneca, which started in March. Perhaps, more importantly, the cause of each serious event has not been determined to be the vaccine in the vast majority of cases.
However, there was one expert, Edwin Michael, Professor of Infectious Disease Epidemiology at the University of South Florida, who said the Jamaican health ministry “should start to track adverse events after the AstraZeneca more carefully, and the age of those experiencing these serious adverse reactions should also be evaluated.” Older adults are naturally more pre-disposed to other illnesses and death.
18º North has since requested to know the breakdown of all serious adverse events by vaccine type and age. In a previous story, 18º North revealed that at least eight people who had died following vaccination, regardless of known cause, were all over 60.
Symptoms For the Serious Adverse Events Not Fleshed Out In Report
One of the limitations of the Oct. 1 report is that it doesn’t fully lay out the symptoms of the 76 serious adverse events. It only categorizes them as death, life threatening, hospitalizations, medically-significant events, disability and congenital abnormalities or birth defects. (There were no birth defects reported.)
The symptoms are important so they can be quickly identified and effectively treated and also because, at the local level, these incidents would then have to be compared against data showing how typical these conditions have been in the population to be able to assess whether there are any significant safety signals that need to be addressed.
Dr. Ennis has said that these local data are sent to the WHO’s drug-safety monitoring database called VigiBase for assessment.
So far, for Jamaica specifically, the public has not heard about any areas of concern that have been flagged.
In various bits of information it put out on the Covid-19 vaccines, the WHO recommends monitoring of serious adverse events following vaccination, including cases of multisystem inflammatory syndrome, thrombosis with thrombocytopenia syndrome (blood clots with low platelet counts), the heart conditions myocarditis and pericarditis, anaphylaxis, other serious allergic reactions, Bell’s palsy, which causes facial muscle weakness or paralysis, and Guillain-Barré syndrome (GBS) - a rare, autoimmune disorder in which a person's own immune system damages the nerves, among other ailments.
4But worldwide, these more serious adverse events have been found to be rare.
For example, in its recommendations for use of the AstraZeneca vaccine, the WHO disclosed that the risk of blood clots with low platelet counts in the U.K. and European Union was estimated at approximately 1 case per 100,000 vaccinated adults with a higher reported risk in younger adults. It said very few cases have been reported from non-European nations despite extensive use of the drug. Dr. Ennis told 18º North she had not seen myocarditis or pericarditis due to the vaccine locally, and she hasn’t responded to an email asking whether she has yet seen the other side effects.
The rare nature of these specific side effects have led health regulators around the world to conclude that the benefits of continuing to administer the vaccines outweigh the risks.
“When you compare taking the vaccine with contracting Covid and being hospitalized, people need to work out the math carefully,” Prof. Peter Figueroa, chair of the Pan American Health Organization’s Technical Advisory Group on Vaccine-Preventable Diseases, told 18º North in June.
Data released by the health ministry on Sept. 30 indicate that of the known cases - which are often fewer than the real numbers - about 5one in every 35 persons has caught Covid in Jamaica. One in every 28 cases that caught the virus was hospitalized. Meanwhile, one in every 45 cases ended up deceased.
Even in a hypothetical scenario, if all the 76 serious adverse events outlined in the ministry’s internal document were found to have been caused by the vaccine it would mean that there was one serious complication recorded for every 9,900 doses approximately. In actuality, Prof. Figueroa suggests that this probability would be overstating the risk as the adverse event reporting system encourages reports of health events that may occur following vaccination even if there is no obvious causal relationship.
“Most of these are obviously not related to the vaccine and most of the remainder are unlikely to be related,” he said.
Video: Chief Medical Officer Dr. Jacquiline Bisasor-McKenzie told journalists on Sept. 30 that the reports of deaths following the vaccine don’t mean that the vaccine caused the death.
The Current State of Play in Jamaica on Vaccination
Presently, only about 15% of Jamaica’s population is fully vaccinated against Covid-19, and the seven-day average for the positivity rate is still hovering around 12% when the desired level is below 5%.
While this reflects an improvement from where the country was in late August and early September when the weekly average positivity rate topped 40%, CMO Dr. Bisasor-McKenzie lamented on Oct. 26, “Our level of vaccination is still low that it is not having a good effect on transmission.”
She said it’s the infection prevention and control methods like restrictions that have been “contributing to the numbers going down.”
But with 98% of Covid deaths occurring in the unvaccinated and fewer proportionate severe cases and deaths in the 60-79 age groups where about 40% of persons have had at least their first dose, she said vaccination has started to have an effect on preventing severe disease and death.
“Everybody needs to get vaccinated. We need to get our numbers up,” she said.
####
Editor’s Notes:
The reported number of deaths between the vaccine rollout on March 10 and Sept. 28, the end of the period that this internal document covered, was 1,385.
There has been one correction. The new insert: “There was a report of an adverse event for every 1,448 cases of the Pfizer; one for every 1,504 of the AstraZeneca and one in every 3,260 of the J&J” is still consistent with the same trend in the earlier version of the story of more adverse events reported after the Pfizer. However, the numbers in the original report were specific to those events classified as non-serious or unknown rather than to the overall number of reports of adverse events.
Footnotes:
The total vaccine doses presented at the Ministry of Health press conference on Sept. 30 of 781,496 differs from the 775,539 doses on its internal dashboard as of that same date. The figures from the internal dashboard also keep updating each time 18º North checks data for the same dates. Overall doses as of Sept. 28 were 752,173 on the internal dashboard.
In the WHO’s manual on assessing causality after immunization, indeterminate temporal means that there was “insufficient definitive evidence” to determine the vaccine caused the event at the time of review. The manual adds that it may be a new vaccine-linked event, and the details of such cases should be maintained in a national database to see if similar events re-occur.
In PAHO’s Guide for the Preparation of a Risk Communication Strategy for COVID-19 Vaccines, there is some guidance that seems to conflict with itself. It states, “Confirm all information before issuing it. Do not talk about assumptions or preliminary information. Relative information leads to discursive confusion.” However, in another PAHO document, Crisis Communication Related to Vaccine Safety: Technical Guidance, in a particular hypothetical scenario where there is a child’s death after immunization and the preliminary information shows that the vaccine was not the cause, it states, “Communicate about the event and the preliminary findings of the investigation as soon as possible.” 18º North asked for clarity from PAHO, but it did not provide a response.
A recent study showed that while the overall incidence of myocarditis, inflammation of the heart, was just over two cases per 100,000 people across all age groups and sexes, nearly 11 of every 100,000 males (1 in every 9,091) in the 16-29 age group developed the condition a few days after having been fully vaccinated. An excerpt from a Aug. 25 New York Times article that mentioned the risk of both myocarditis and pericarditis, which is inflammation of the lining around the heart, also stated, “In their review of the Pfizer-BioNTech vaccine, regulators paid close attention to an American health care claims database, which found that the risk of the conditions in 16- and 17-year-old vaccinated boys could be as high as 1 in 5,000. The cases in the database were unconfirmed, the F.D.A. cautioned in an analysis published this week, but they were considered a reasonable estimate of the possible risk. Even in the worst-case scenarios of post-vaccination myocarditis and pericarditis modeled by the F.D.A., the benefits of vaccination still outweighed the risks, the analysis said.” Indeed, another study showed that the risk of myocarditis and pericarditis was higher after Covid-19 than after vaccination.
Per person estimates are approximate as number of cases don’t necessarily equate to number of persons. Some persons have been diagnosed with Covid-19 more than once.
You’ve gotten this far. Congratulations! You must really be into this story from 18º North. Consider becoming a paid subscriber and help us write more articles like this one that hold the government to account. It’s US$11.50 a month or US$115 a year, including sales tax.